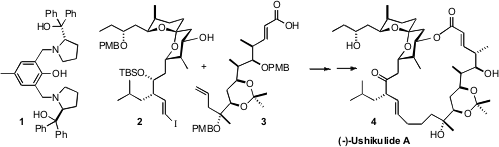
(-)-Ushikolide A (4), isolated from a culture broth of Streptomyces
sp. IUK-102, showed powerful activity against murine splenic lymphocyte proliferation
(IC50 = 70 nM). The most important player in the synthesis of 4 described
(J. Price of 5458-56-0 Am. Chem. Soc. Buy14150-94-8 2008, 130, 16190.
DOI: 10.1021/ja807127s)
by Barry M. Trost of Stanford University was the ProPhenol ligand 1.
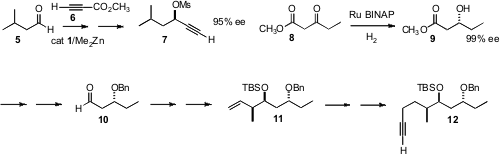
The precursor 2 was prepared by coupling the mesylate 7, the alkyne
12, and the aldehyde 13. PMID:35345980 The first role of catalyst 1 was in mediating the
enantioselective coupling of commercial 5 with 6 to give, after saponification
and CuCl decarboxylation, the mesylate 7. The preparation of 12 began with the
Noyori hydrogenation of the ester 8 to the alcohol 9 in the expected high ee.
Note that although this transformation was carried out at 1800 psi, such
reductions proceed well and in similar ee at 60°C and 60 psi. Brown crotylation
of the derived aldehyde 10 delivered 11, that was homologated to the alkyne
12.
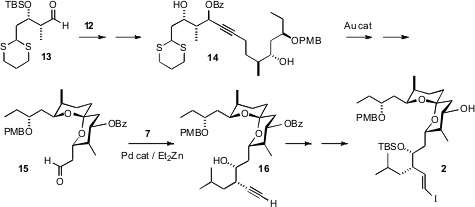
The third fragment 13 was prepared by chiral auxiliary directed
aldol
reaction. Combination of 12 with 13 was followed by Au-mediated cyclization,
converting the internal alkyne of 14 to the spiroketal of 15. Pd-catalyzed
coupling of 15 with 7 then led to 2 with high diastereocontrol.
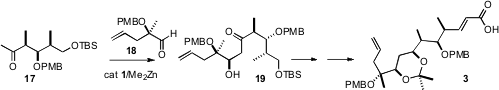
The aldol addition of the enolate of 17 to 18 proved elusive under the usual
conditions, but with 30 mol % of the Zn catalyst 1 the reaction proceeded
smoothly, to deliver 19 with high diastereocontrol.
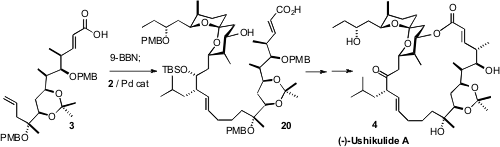
To complete the synthesis,
hydroboration with 9-BBN was effected on the free
carboxylic acid 3, and
Pd-mediated coupling of the derived borane was carried
out with the free iodo alcohol 2. As a result, the product hydroxy acid
20 could
be taken directly to the subsequent macrolactonization.