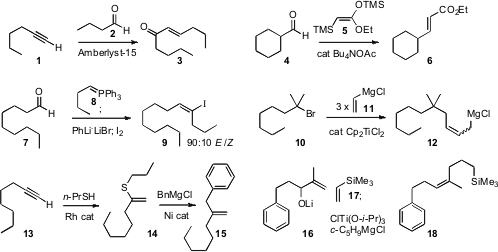
Products such as 3 and 6 are usually prepared by phosphonate
condensation. J. S. PMID:23756629 Yadav of the Indian Institute of Technology, Hyderabad found
(Tetrahedron Lett. 2008, 49, 4498.
DOI: 10.1016/j.tetlet.2008.05.056)
that the cation-exchange resin Amberlyst-15 in CH2Cl2 mediated
the condensation of a terminal alkyne such as 1 with an aldehyde to give the
enone 3. Similarly, Teruaki Mukaiyama of Kitasato University showed
(Chem. Lett. 2008, 37, 704.
DOI: 10.1246/cl.2008.704)
that tetrabutylammonium acetate mediated the
condensation of 5 with an aldehyde such as 4 to give the ester
6. David M. Fmoc-Lys(Mtt)-OH structure Hodgson of the University of Oxford described
(J. Am. Chem. Soc. N-Boc-4-pentyne-1-amine In stock 2008, 130, 16500.
DOI: 10.1021/ja8076999)
the optimization of the
Schlosser protocol for the condensation of a phosphorane with an aldehyde 7
followed by deprotonation and halogenation, to deliver the alkenyl halide 9
with good geometric control. Jun Terao of Kyoyo University and Nobuaki Kambe of
Osaka University accomplished
(Chem. Commun. 2008, 5836.
DOI: 10.1039/b813596g)
the homologation of a halide such as 10 to the corresponding allylic
Grignard
reagent 12. Primary, secondary and tertiary halides worked well. Jennifer
Love of the University of British Columbia developed
(Org. Lett. 2008, 10, 3941.
DOI: 10.1021/ol8012843)
a Rh catalyst for the addition of thiols to terminal alkynes
such as 13, and found that the product thioether 14 coupled
smoothly with Grignard reagents to deliver the 1,1-disubstituted alkene 15.
Glenn C. Micalizio, now at Scripps Florida, established
(J. Am. Chem. Soc. 2008, 130, 16870.
DOI: 10.1021/ja8074242)
what appears to be a general method for the
construction of Z-trisubstituted alkenes such as 18.
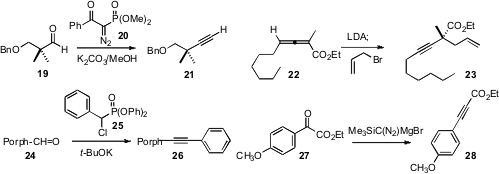
The Ohira protocol has become the method of choice for converting an aldehyde
19 to the alkyne 21. We have found (Tetrahedron Lett.
2008, 49, 6904.
DOI: 10.1016/j.tetlet.2008.09.114)
that the reagent 20 offers advantages in price,
preparation and handling. Bo Xu and Gerald B. Hammond of the University of
Louisville observed
(Org. Lett. 2008, 10, 3713
DOI: 10.1021/ol801287t)
that an allene ester such as 22 is readily homologated to the alkyne 23.
Ashton C. Partridge of Massey University extended
(Tetrahedron Lett. 2008, 49, 5632.
DOI: 10.1016/j.tetlet.2008.07.059)
condensation with the aryl phosphonate 25 to
porphyrin aldehydes, leading to alkynes such as 26. Toyohiko Aoyama of
Nagoya City University established
(Tetrahedron Lett. 2008, 49, 4965.
DOI: 10.1016/j.tetlet.2008.05.155)
that the halomagnesium salt of TMS diazomethane was the most efficient for
converting 27 to 28 [CAUTION: Two deaths were recently reported
from use of TMS diazomethane without proper ventilation!].
Masahiru Miura, also of Osaka University, reported
(Chem. Commun. 2008, 3405.
DOI: 10.1039/b804573a)
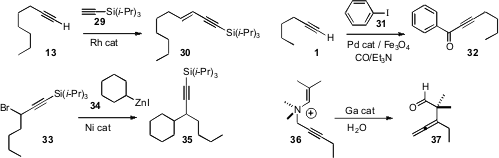
that the Rh catalyzed homologation of a terminal alkyne 13
to the enyne 30 proceeded with high geometric control. Wei Sun and Chungu
Xia of the Lanzhou Institute of Chemical Physics devised
(Org. Lett. 2008, 10, 3933.
DOI: 10.1021/ol801478y)
a magnetically-retrievable Pd catalyst, the utility
of which they illustrated with the carbonylative
Sonogashira coupling of 1
with 31 to give 32. Gregory C. Fu of MIT optimized
(Angew. Chem. Int. Ed. 2008, 47, 9334.
DOI: 10.1002/anie.200802784)
the Ni catalyzed coupling of organozinc reagents such as 34
with a propargylic halide 33 to give 36.
In an elegant extension of their “nanozyme” work, Robert G. Bergman and
Kenneth N. Raymond of the University of California, Berkeley devised
(J. Am. Chem. Soc. 2008, 130, 10977.
DOI: 10.1021/ja8013055)
an encapsulating Ga complex that catalyzed the rearrangement of 36 to
the allene 37. Depending on how well the quaternary ammonium salt fit
in the encapsulating complex, they observed rate accelerations of more than
two orders of magnitude for the rearrangement.