B (de la Pradilla), Sanguiin H-5 (Spring), Solandelactone A (White),
Spirastrellolide A (Paterson)
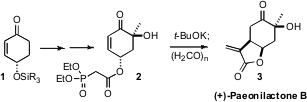
Richard J. K. Taylor of the University of York has developed
(Angew. Buy2422999-74-2 Chem. Int. Ed. PMID:23892407 2008, 47, 1935.
DOI: 10.1002/anie.200705329)
the diasteroselective intramolecular
Michael cyclization of phosphonates such as 2.
Quenching of the cyclized product with paraformaldehyde delivered (+)-Paeonilactone B (3).
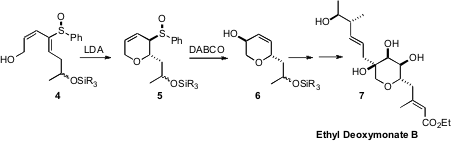
Roberto Fernández de la Pradilla of the CSIC, Madrid established
(Tetrahedron Lett. 2008, 49, 4167.
DOI: 10.1016/j.tetlet.2008.04.107)
the diastereoselective intramolecular hetero Michael addition of alcohols to enantiomerically-pure
acyclic sulfoxides such as 4 to give the allylic sulfoxide 5. Fmoc-D-beta-indanylglycine custom synthesis
Mislow-Evans rearrangement converted 5 into 6, the
enantiomerically-pure core of Ethyl Deoxymonate B (7).
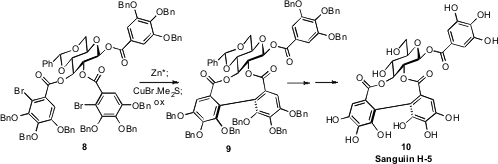
The ellagitannins, represented by 10, are single atropisomers around
the biphenyl linkage. David R. Spring of the University of Cambridge found
(Org. Lett. 2008, 10, 2593.
DOI: 10.1021/ol8009545)
that the chiral constraint of the carbohydrate backbone of 9 directed the absolute sense of the oxidative
coupling of the mixed cuprate derived from 9, leading to Sanguiin H-5 (10)
with high diastereomeric control.
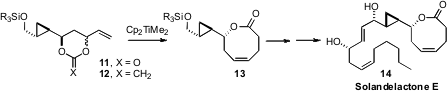
A key challenge in the synthesis of the solandelactones, exemplified by 14,
is the stereocontrolled construction of the unsaturated eight-membered ring
lactone. James D. White of Oregon State University found
(J. Org. Chem. 2008, 73, 4139.
DOI: 10.1021/jo800335g)
an elegant solution to this problem, by exposure of the cyclic carbonate 11 to the Petasis reagent, to give 12.
Subsequent Claisen rearrangement delivered the eight-membered ring lactone, at
the same time installing the ring alkene of Solandelactone E (14).
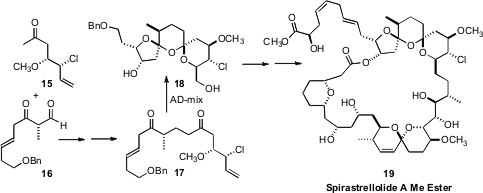
AD-mix usually proceeds with only modest enantiocontrol with terminal
alkenes. None the less, Ian Paterson, also of the University of Cambridge,
observed (Angew. Chem. Int. Ed. 2008, 47, 3016,
DOI: 10.1002/anie.200705565;
Angew. Chem. Int. Ed. 2008, 47, 3021,
DOI: 10.1002/anie.200705566)
that bis-dihydroxylation of the diene 17 proceeded to give, after acid-mediated cyclization, the
bis-spiro ketal core 18 of Spirastrellolide A Methyl Ester (19)
with high diastereocontrol.