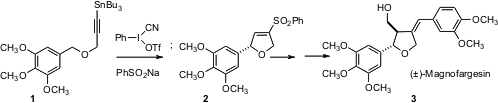
Duncan J. Wardrop of the University of Illinois, Chicago, established (Org. 529476-80-0 Chemical name Lett. 2006, 8, 3659.DOI: 10.1021/ol0609053)the five-membered ether ring of (±)-magnofargesin (3) by the addition of benzenesulfinate to the activated alkyne derived from 1. The alkylidene carbene so generated then inserted into the C-H bond adjacent to the ether oxygen, to give 2.
Six-membered ring cyclic ethers, in particular 2,6-dialkyl tetrahydropyrans, are ubiquitous in natural products. Several of the Annonacae acetogenins show remarkable antitumor activity. (-)-Jimenezin (6) is unusual in that it contains a six-membered ring. In the course of a synthesis of 6, Reinhard W. Hoffmann and Ulrich Koert of the Philipps-Universität Marburg (Org. PMID:24578169 Cholesterol Chemical name Lett. 2006, 8, 3829. DOI: 10.1021/ol0614471)assembled that ring by cyclization of the allyl borinate 4 to 5 with high diastereocontrol.
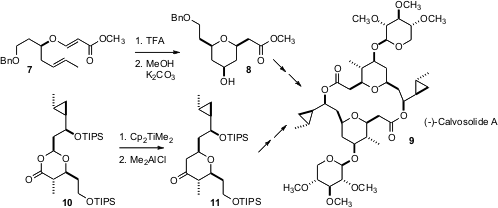
Both Christine Willis of the University of Bristol (Org. Lett. 2006, 8, 3319. DOI: 10.1021/ol0611705)and Amos B. Smith III of the University of Pennsylvania (Org. Lett. 2006, 8, 3315. DOI: 10.1021/ol0611752)have reported syntheses of the dimeric (-)-calvosolide A (9). In the Willis synthesis, the2,6-dialkyl tetrahydropyran was prepared by the acid-catalyzed (“Prins”) cyclization of 7 to 8. In the Smith synthesis, the 2,6-dialkyl tetrahydropyran was prepared by the Petasis-Ferrier rearrangement of 10 to 11.
In each case, completion of the synthesis of (-)-calvosolide A (9) involved macrolide construction by the Yamaguchi method, formation of the mixed anhydride with 2,4,6-trichlorobenzoyl chloride. Extending macrolactonization to larger rings, to prepare 14, a component of the defensive secretion of a soldier termite, Isamu Shiina of the Tokyo University of Science found (Org. Lett. 2006, 8, 4955. DOI: 10.1021/ol062011o)that a modified protocol using the anhydride of 2-methyl-6-nitrobenzoic acid was more effective.
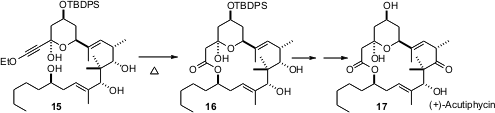
Timothy F. Jamison of MIT has put forward (J. Am. Chem. Soc. 2006, 128, 15106. DOI: 10.1021/ja0670660)a complementary method for macrolide formation, pyrolysis of the ethoxy acetylene 15, to prepare (+)-acutiphycin 17. This method for ketene generation was originally developed by Raymond L. Funk.