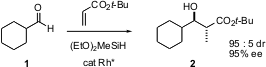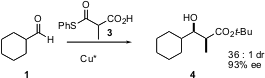There are several strategies now available for the catalytic construction of isolated oxygenated and aminated stereogenic centers in high enantiomeric excess (![]() 2006 Jan 9). Processes for the catalytic enantioselective construction of extended arrays of aminated, oxygenated and alkylated stereogenic centers have also been developed (
2006 Jan 9). Processes for the catalytic enantioselective construction of extended arrays of aminated, oxygenated and alkylated stereogenic centers have also been developed (![]() 2005 June 27).
2005 June 27).
The enantioselective element can be complexed with the nucleophile, with the acceptor, or both. A powerful approach is to use the chiral ligand sphere around a catalytic transition metal as the controlling element. Hisao Nishiyama of Nagoya University has recently (J. Am. Chem. (S)-BI-DIME Purity Soc. 2005, 127, 6972. 4-Bromo-5-fluoropyridin-2-amine manufacturer DOI: 10.1021/ja050698m)developed such an approach. PMID:23443926 Silane-mediated reduction of an unsaturated ester in the presence of an aldehyde and a chiral Rh catalyst delivered the anti aldol product 2 in high diastereoselectivity and enantioselectivity.
In a complementary approach, Matthew D. Shair of Harvard University has found (J. Am. Chem. Soc. 2005, 127, 7284.DOI: 10.1021/ja051759j)that Cu*-catalyzed decarboxylation of malonate thioesters such as 3 in the presence of an aldehyde led to the syn aldol product 4 in high diastereoselectivity and enantioselectivity.
Enantomerically-pure secondary amines can condense with reactive ketones to make enantiomerically-pure enamines. Essentially simultaneously, Dieters Enders at the RTWH, Aachen, (Angew. Chem. Int. Ed. 2005, 44, 1210.DOI: 10.1002/anie.200462428)and Carlos F. Barbas III at Scripps (Org. Lett. 2005, 7, 1383.DOI: 10.1021/ol0502533)described the highly diastereoselective proline-catalyzed condensation of the acetonide 5 of dihydroxy acetone with aldehydes such as 6 to give the differentially-protected anti aldol product 7 in high ee.
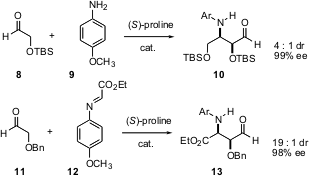
In a remarkable series of three papers, Armando Córdova of Stockholm University has expanded on the proline-mediated condensation of activated aldehydes such as 8. In the first paper (Tetrahedron Lett. 2005,46, 2839.DOI: 10.1016/j.tetlet.2005.02.116), enantioselective Mannich reaction is described. Homocondensation with an aryl amine 9 gave the four-carbon array 10 in high ee. Condensation with other activated imines such as 12 also proceeded efficiently.
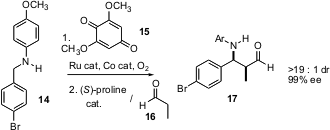
In the following paper (Tetrahedron Lett. 2005, 46, 3363. DOI: 10.1016/j.tetlet.2005.03.084), Professor Córdova reported that condensations such as 5 with 6 (above) proceed more quickly and in higher yield and ee when a small quantity of water is deliberately added to the reaction mixture. He also reported the proline-catalyzed Mannich condensation of 5. In the final paper of the series, (Tetrahedron Lett. 2005, 46, 3965.DOI: 10.1016/j.tetlet.2005.04.047), co-authored by Jan E. Bäckvall, Professor Córdova reported oxidation of benzylic amines such as 14 to the imine, with subsequent in situ Mannich condensation with 16 to give 17.