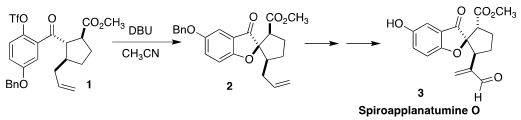
Spiroapplanatumine O (3) was isolated from Ganoderma applantum,
a wood-decaying fungus that is widely used in traditional medicinal chemistry.
Ullrich Jahn of the Institute of Organic Chemistry and Biochemistry of the Czech
Academy of Sciences assembled the ketone 2 via the cyclization of the
aryl triflate 1
(Org. PMID:25040798 Lett. 349552-70-1 web 2022, 24, 4552.
DOI: 10.1021/acs.orglett.2c01633). 3,4-Diaminobenzenesulfonic acid supplier
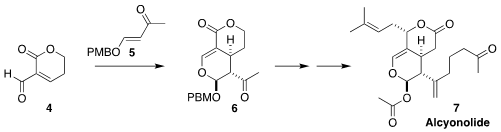
Alcyonolide (7), isolated from a broth of an Okinawan soft coral of
the genus Alcyonium, showed activity against HCT116 and NBT-T2 cells.
Seijiro Hosokawa of Waseda University prepared the bicyclic core 6 of
7 by the hetero Diels-Alder addition of the alkoxy enone 5 to the
unsaturated aldehyde 4
(J. Org. Chem. 2022, 87, 15492.
DOI: 10.1021/acs.joc.2c02031).
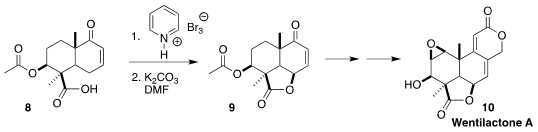
Wentilactone A (10), isolated from the mold Aspergillus wentii
EN-48, induced G2/M phase arrest and the mitochondrial-related apoptosis of lung
cancer cells. En route to 10, Peng Xu and Biao Yu of SIOC cyclized the
acid 8 to the lactone 9
(Chem. Commun. 2022, 58, 12487.
DOI: 10.1039/D2CC04930A).
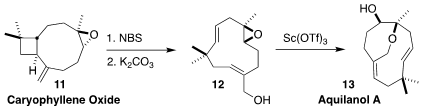
Aquilanol A (13) was isolated from the agarwood of Aquilaria
malaccensis, produced in the heartwood of the tree on infection by the
fungus Phialophora parasitica. Christos I. Stathakis of the Aristotle
University of Thessaloniki effected bromination and elimination of caryophyllene
oxide (11) to give the allylic alcohol 12, then cyclized that to 13
(Org. Lett. 2022, 24, 6242.
DOI: 10.1021/acs.orglett.2c02216).
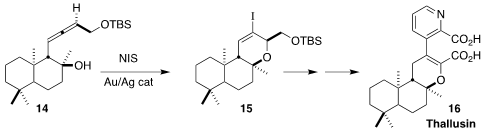
Thallusin (16), isolated from the bacterium Cytophaga sp.
strain YM2-23, induces stable thalus formation in the chlorophyte Gayralia
oxyspermum. Hans-Dieter Arndt of the Friedrich-Schiller-Universität Jena
established the
dihydropyran ring of 16 by the Au/Ag-promoted
iodocyclization of the allene 14 to 15
(Angew. Chem. Int. Ed. 2022, 61, e202206746.
DOI: 10.1002/anie.202206746).
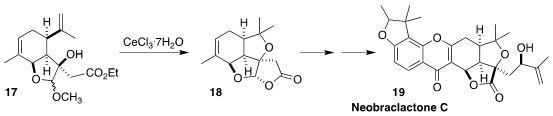
Neobraclactone C (17) was isolated from the leaves of the Chinese
tropical tree Garcinia anomala along with neobraclactones A and B. The
latter showed pronounced growth inhibitory activity against the human leukemia
cell line K562 and HL-60. Maosheng Cheng and Yongxiang Liu of Shenyang
Pharmaceutical University assembled the lactone 18 by the cyclization of
the carvone-derived alcohol 17
(Org. Lett. 2022, 24, 9485.
DOI: 10.1021/acs.orglett.2c03970).