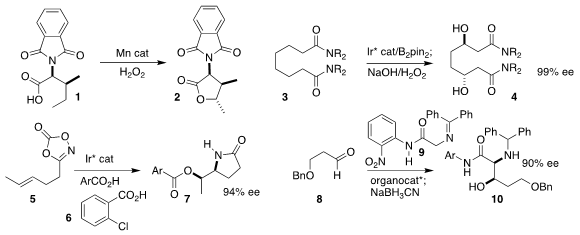
Massimo Bietti of the Università "Tor Vergata" and Miquel Costas of the
Universtat de Girona achieved substantial diastereoselectivity in the oxidative
cyclization of the carboxylic acid 1 to the
lactone 2
(Angew. Chem. Int. Methyl 4-hydroxyphenylacetate custom synthesis Ed. 2021, 60, 4740.
DOI: 10.1002/anie.202007899).
Senmiao Xu of the Lanzhou Institute of Chemical Physics effected the enantioselective
β-hydroxylation of the bis-amide 3, leading to the 1,4-diol 4
(Angew. Formula of 849020-87-7 Chem. Int. Ed. 2021, 60, 3524.
DOI: 10.1002/anie.202013568).
Seung Youn Hong and Sukbok Chang of KAIST prepared the
lactam
7 by coupling the isoxazolone 5 with the carboxylic acid 6
(J. Am. PMID:30125989 Chem. Soc. 2021, 143, 3993.
DOI: 10.1021/jacs.1c00652).
Antonia Mielgo and Claudio Palomo of the Universidad del País Vasco used a ureidopeptide-based
Brønsted base to prepare the amino alcohol 10 by the addition of the protected
glycine 9 to the aldehyde 8
(J. Org. Chem. 2021, 86, 7757.
DOI: 10.1021/acs.joc.1c00406).
Lei Zhang of the South China University of Technology and Zhongqing Wang of HEC Pharm Group
described the enantioselective reduction of the enol 11 to the lactam 12
(Adv. Synth. Catal. 2021, 363, 3030.
DOI: 10.1002/adsc.202100288).
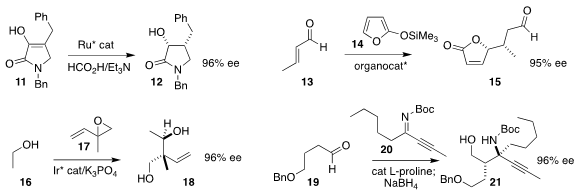
Patrick G. Harran of UCLA used the Hayashi-Jørgensen catalyst to mediate the enantioselective
conjugate addition of the silyl enol
ether 14 to crotonaldehyde 13, leading to the lactone 15
(Tetrahedron Lett. 2021, 72, 153056.
DOI: 10.1016/j.tetlet.2021.153056).
Michael J. Krische of the University of Texas established conditions for the coupling of
ethanol 16 with racemic isoprene monoepoxide 17
to give the diol 18
(Angew. Chem. Int. Ed. 2021, 60, 10542.
DOI: 10.1002/anie.202102694).
Taichi Kano and Keiji Maruoka of Kyoto University assembled the amino
alcohol 21 by the addition of the aldehyde 19 to the imine 20
(Chem. Sci. 2021, 12, 1445.
DOI: 10.1039/D0SC05269H).
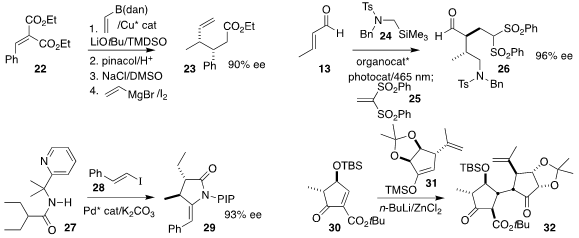
Jaesook Yun of Sungkyunkwan University showed that the intermediate from the
enantioselective conjugate addition of a boroalkene to the alkylidene malonate
22 could be carried on to the ester 23
(Angew. Chem. Int. Ed. 2021, 60, 4614.
DOI: 10.1002/anie.202014425).
Paolo Melchiorre of ICIQ used a gemdifluoro diaryl prolinol catalyst to mediate
the photochemically-promoted three-component assembly of the aldehyde 26 by the
coupling of 13 and 24 with 25
(Angew. Chem. Int. Ed. 2021, 60, 5357.
DOI: 10.1002/anie.202014876).
Ye-Qiang Han of Zhejiang University and Bing-Feng Shi of Zhengzhou University observed
high diastereoselectivity in the preparation of the lactam 29 by the coupling of
the amide 27 with the alkenyl iodide 28
(Org. Lett. 2021, 23, 2048.
DOI: 10.1021/acs.orglett.1c00204).
Raphaël Oriez of BIKAKEN and Joëlle Prunet of the University of Glasgow also achieved
high diastereoselectivity in the assembly of 32 by the conjugate addition of the
silyl enol ether 31 to the
cyclopentenone 30
(Tetrahedron 2021, 79, 131843.
DOI: 10.1016/j.tet.2020.131843).
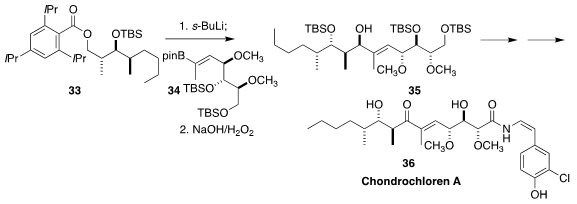
Chondrochloren A (36), isolated from the myxobacterium Chondromyces crocatus,
showed weak antibiotic activity. In the course of a synthesis of 36, Markus Kalesse of the Gottfried Wilhelm Leibniz Universität Hannover observed
remarkable diastereoselectivity in the coupling of 33 with 34 to give the
allylic alcohol 35
(Angew. Chem. Int. Ed. 2021, 60, 6938.
DOI: 10.1002/anie.202016072).