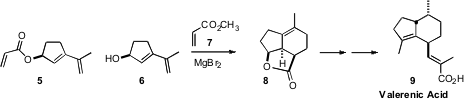It has generally been observed that prospective intramolecular
Diels-Alder
cycloadditions that would form a γ-lactone are reluctant to proceed. 5-Ethynylpyridine-2-carbaldehyde web In the
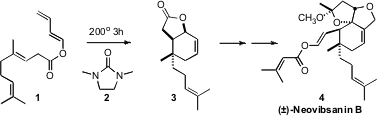
course of a synthesis of (±)-Neovibsanin B (4), Hiroshi Imagawa and Mugio
Nishizawa of Tokushima Bunri University reversed
(Org. Lett. 2009, 11, 1253.
DOI: 10.1021/ol802973f)
the usual connectivity, and found that the dienyl ester 1 could be induced to
cyclize to 3. The solvent 2 improved both the yield and the diastereoselectivity
of the cycloaddition. 4-Bromo-2-methylpyrimidine Chemscene
It was not surprising that Johann Mulzer of the University of Vienna could see
(Org. Lett. PMID:23489613 2009, 11, 1151.
DOI: 10.1021/ol9000137)
no evidence of cyclization on heating the acrylate 5. In contrast, following the lead of Barriault,
they found that MgBr2-tethered cycloaddition of methyl acrylate (7) with the alcohol
6 proceeded smoothly, to give 8, that they carried on to Valerenic Acid (9).
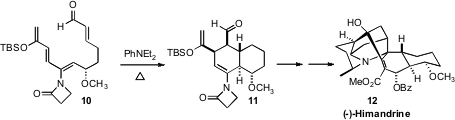
Intramolecular Diels-Alder reactions to form 6,6-systems are often facile.
Mohammad Movassaghi of MIT, en route to (-)-Himandrine (12), showed
(J. Am. Chem. Soc. 2009, 131, 9648.
DOI: 10.1021/ja903790y)
that the tetraene 10 cyclized to 11 at 95°C with 5:1 dr.
In this case the solvent was the more typical acetonitrile with 10% diethyl
aniline.
The intramolecular Diels-Alder reaction is concerted but non-synchronous
(Tetrahedron Lett. 1981, 22, 5141.
DOI: 10.1016/S0040-4039(01)92442-6),
with β-bond formation preceding α-bond formation. This
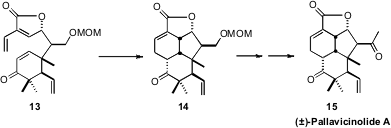
contributes to the reluctance of 8 to cyclize. In contrast, Henry N. C. Wong of
the Chinese University of Hong Kong observed
(Angew. Chem. Int. Ed. 2009, 48, 2351.
DOI: 10.1002/anie.200806335)
that the tetraene 13, that is polarity matched, cyclized to
14 spontaneously as soon as it was formed. Functional group conversion completed
the synthesis of (±)-Pallavicinolide A (15).
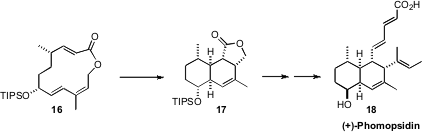
The transannular Diels-Alder (TADA) reaction can proceed with high
diastereocontrol, but the factors directing these cyclizations are not
completely understood. In the course of a synthesis of (+)-Phomopsidin (18),
Masahisa Nakada of Waseda University found
(Tetrahedron 2009, 65, 888.
DOI: 10.1016/j.tet.2008.11.030)
that 16 led to 17 with 16:1 dr.
In contrast, the triene epimeric to 16 at the silyloxy
group cyclized with only 2:1 dr.