The radical chemistry associated with organosilanes has been the subject of my recent book (Chatgilialoglu C. Organosilanes in Radical Chemistry; Wiley: Chichester, 2004). 7-Chloropyrido[3,4-b]pyrazine In stock In particular, the use of(TMS)3SiH in organic synthesis has been treated in some details with numerous examples. PMID:24140575 Starting from this month, I will describe some more recent examples of the use of (TMS)3SiH in organic synthesis that are not included in my book.
A radical carboxyarylation approach is introduced by Sherburn and coworkers in two communications as the key step in the total synthesis of several biologically important natural products (J. Am. Chem. Soc. 4-Bromo-2-ethylpyridine structure 2003, 125, 12108,DOI: 10.1021/ja0376588; Org. Lett. 2004, 6, 1345,DOI: 10.1021/ol049878b). Treatment of thiocarbonate derivatives 1 (R = Me or TBS) with 1.1 equiv of (TMS)3SiH in refluxing benzene and in the presence of AIBN (0.4 equiv added over 6 h) as radical initiator, produced compound 2 in 44% yield. This remarkable transformation resulted from a radical cascade, involving (TMS)3Si. radical addition to thiocarbonyl function (1->3), 5-exo cyclization (3->4) and intramolecular 1,5-ipso substitution (4->5) with the final ejection of (TMS)3SiS. radical.
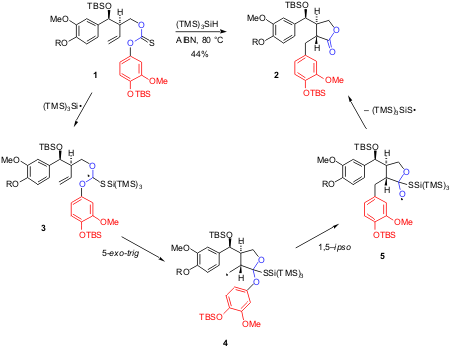
As a personal mechanistic remark, I suggest that the ejected thiyl radical undergoes a fast 1,2-migration of silyl group from silicon to sulfur (Reaction 1), affording a new silyl radical that either reacts with (TMS)3SiH (Reaction 2) completing the reaction cycle, or replaces the (TMS)3Si. radical in the above described reaction sequence.
![]()
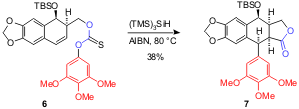
This effective route has also been applied to structurally analogous thiocarbonate 6 that affords 7 in 38% yield. Although the reaction sequence is the same, it is worth underlining that the resulting carbon-centered radical from the 5-exo cyclization is a benzylic-type, which is still reactive towards the 1,5-ipso substitution.